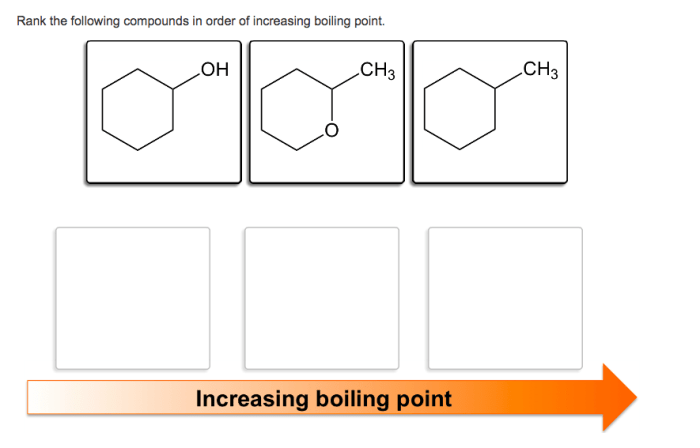
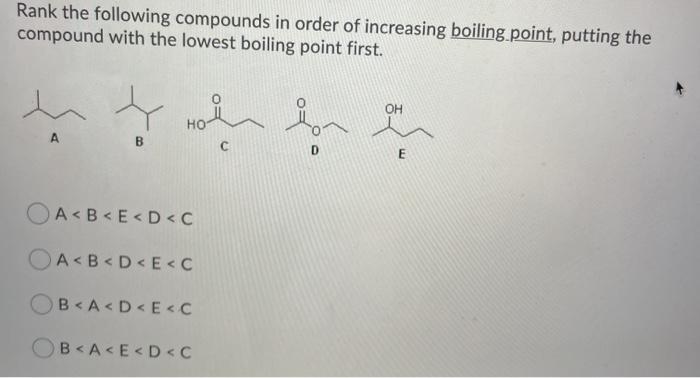
Rank the compounds in order of increasing boiling point – Boiling point is a fundamental property of compounds that plays a crucial role in various chemical processes. This article delves into the relationship between boiling point and intermolecular forces, providing a comprehensive guide to ranking compounds in order of increasing boiling point.
The boiling point of a compound is directly influenced by the strength of intermolecular forces. Stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point. Conversely, weaker intermolecular forces lead to lower boiling points.
Boiling Point and Intermolecular Forces: Rank The Compounds In Order Of Increasing Boiling Point
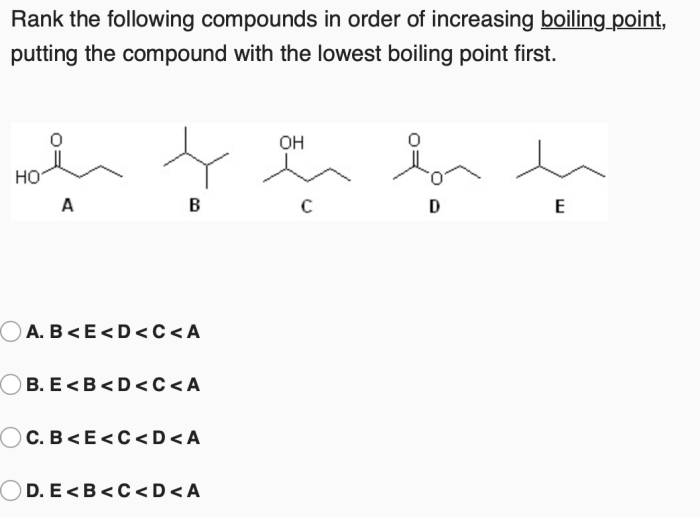
The boiling point of a compound is the temperature at which its vapor pressure equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a compound is influenced by the strength of the intermolecular forces between its molecules.
There are three main types of intermolecular forces: dipole-dipole forces, hydrogen bonding, and London dispersion forces.
- Dipole-dipole forcesoccur between molecules that have permanent dipoles. The strength of the dipole-dipole force depends on the magnitude of the dipole moment and the distance between the molecules.
- Hydrogen bondingis a special type of dipole-dipole force that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. Hydrogen bonding is stronger than dipole-dipole forces.
- London dispersion forcesare weak attractive forces that occur between all molecules. The strength of the London dispersion force depends on the polarizability of the molecule and the number of electrons in the molecule.
The stronger the intermolecular forces between the molecules of a compound, the higher its boiling point.
Ranking Compounds by Boiling Point
The following table lists the boiling points of some common compounds.
| Compound | Boiling Point (°C) |
|---|---|
| Methane | |
| Ethane | |
| Propane | |
| Butane | |
| Pentane | |
| Hexane | |
| Heptane | |
| Octane | |
| Nonane | |
| Decane |
The boiling point of a compound increases with increasing molecular weight.
This is because the stronger the intermolecular forces between the molecules of a compound, the higher its boiling point.
Structural Features and Boiling Point, Rank the compounds in order of increasing boiling point
The structural features of a compound can also affect its boiling point. For example, compounds with a branched structure have lower boiling points than compounds with a straight-chain structure. This is because the branched structure reduces the surface area of the molecule, which in turn reduces the strength of the intermolecular forces between the molecules.
Compounds with a polar structure also have higher boiling points than compounds with a nonpolar structure. This is because the polar structure creates a permanent dipole moment, which strengthens the intermolecular forces between the molecules.
Exceptions and Anomalies
There are some exceptions to the general trend of boiling point increasing with molecular weight or polarity. For example, water has a higher boiling point than ethanol, even though ethanol has a higher molecular weight and a polar structure. This is because water molecules can form hydrogen bonds, which are stronger than the dipole-dipole forces between ethanol molecules.
FAQ
What are the different types of intermolecular forces?
Intermolecular forces include dipole-dipole interactions, hydrogen bonding, van der Waals forces, and ion-dipole interactions.
How does molecular weight affect boiling point?
In general, heavier molecules have higher boiling points due to stronger intermolecular forces.
What is the relationship between polarity and boiling point?
Polar molecules have stronger intermolecular forces, resulting in higher boiling points compared to nonpolar molecules.