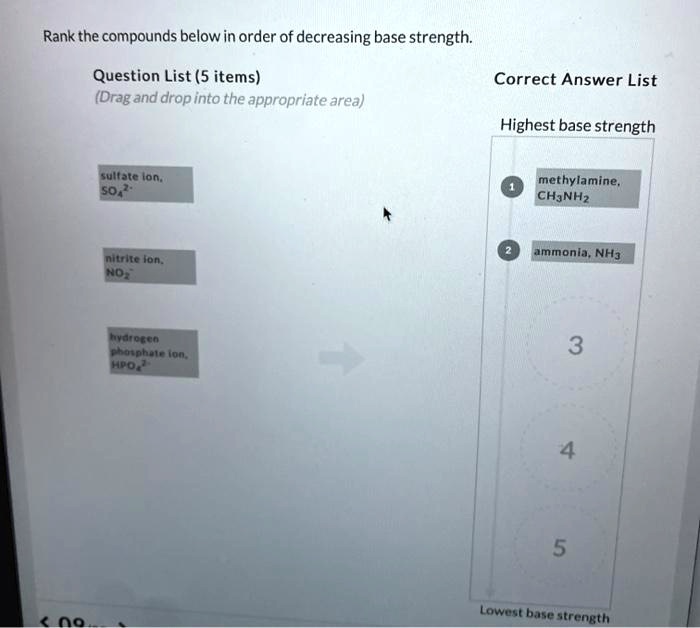
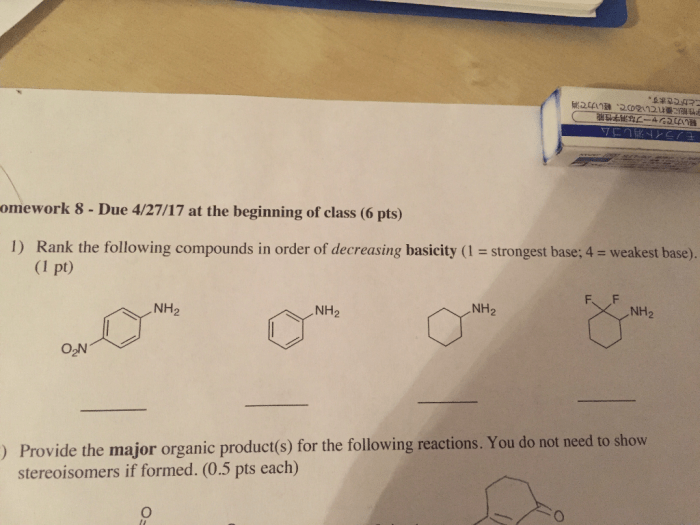
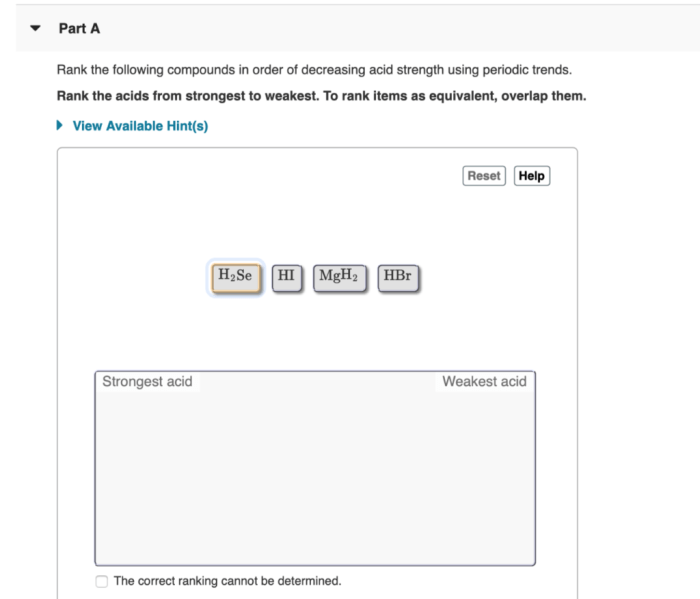
Rank the compounds below in order of decreasing base strength. – In chemistry, understanding the strength of bases is crucial for comprehending various chemical reactions and processes. This guide explores the concept of base strength and provides a comprehensive methodology for ranking compounds based on their decreasing base strength. By examining factors that influence base strength and exploring different ranking methods, we delve into the practical applications of this knowledge in fields such as chemistry, biology, and medicine.
Ranking Compounds by Base Strength: Rank The Compounds Below In Order Of Decreasing Base Strength.

Base strength, a crucial property in chemistry, measures the ability of a compound to accept protons (H +ions). Understanding base strength is essential for predicting and controlling chemical reactions, particularly those involving acid-base interactions.
Ranking compounds based on decreasing base strength involves comparing their relative abilities to accept protons. This ranking is useful for predicting the outcome of acid-base reactions and designing synthetic strategies.
Factors Affecting Base Strength, Rank the compounds below in order of decreasing base strength.
Several factors influence the base strength of a compound:
- Molecular Structure:The arrangement of atoms within a molecule can affect base strength. Generally, compounds with more electronegative atoms (e.g., nitrogen, oxygen) are stronger bases.
- Hybridization:The hybridization of the atom accepting the proton influences base strength. Compounds with sp 3hybridized atoms are typically stronger bases than those with sp 2or sp hybridized atoms.
- Electronegativity:The electronegativity of the atom accepting the proton affects base strength. More electronegative atoms make weaker bases.
Methods for Ranking Compounds
Various methods are used to rank compounds based on base strength:
- pKa Values:The pKa value, which measures the acidity of the conjugate acid of a base, can be used to infer base strength. Lower pKa values indicate stronger bases.
- Aqueous Solubility:The aqueous solubility of a base can provide an indication of its strength. Stronger bases are typically more soluble in water.
- Reaction Rates:The rate of reaction of a base with an acid can also be used to rank base strength. Faster reaction rates indicate stronger bases.
Examples and Applications
Examples of compounds ranked in order of decreasing base strength include:
- Hydroxide ion (OH –) > Amide ion (NH 2–) > Alkoxide ion (RO –)
- Methylamine (CH 3NH 2) > Ethylamine (C 2H 5NH 2) > Propylamine (C 3H 7NH 2)
Base strength ranking has practical applications in various fields:
- Chemistry:Predicting and controlling acid-base reactions, designing synthetic strategies.
- Biology:Understanding enzyme catalysis, maintaining pH homeostasis.
- Medicine:Developing drugs, designing drug delivery systems.
FAQ Resource
What factors influence the base strength of a compound?
Factors such as molecular structure, hybridization, electronegativity, and the presence of electron-withdrawing or electron-donating groups all impact the base strength of a compound.
What is the pKa value and how is it related to base strength?
The pKa value represents the negative logarithm of the acid dissociation constant and is inversely proportional to base strength. A lower pKa value indicates a stronger base.
How can base strength ranking be applied in practical settings?
Base strength ranking finds applications in various fields, including the design of drugs, optimization of chemical reactions, and understanding biological processes.