Dehydration mechanism of 4 methylcyclohexanol – The dehydration mechanism of 4-methylcyclohexanol is a captivating chemical process that unveils the intricate interplay of molecular rearrangements and energy transformations. This mechanism holds significant implications in various scientific disciplines, from organic chemistry to industrial applications. Delving into the depths of this reaction, we will explore its intricate steps, influencing factors, and practical applications, providing a comprehensive understanding of this fundamental chemical process.
Dehydration Mechanism of 4-Methylcyclohexanol
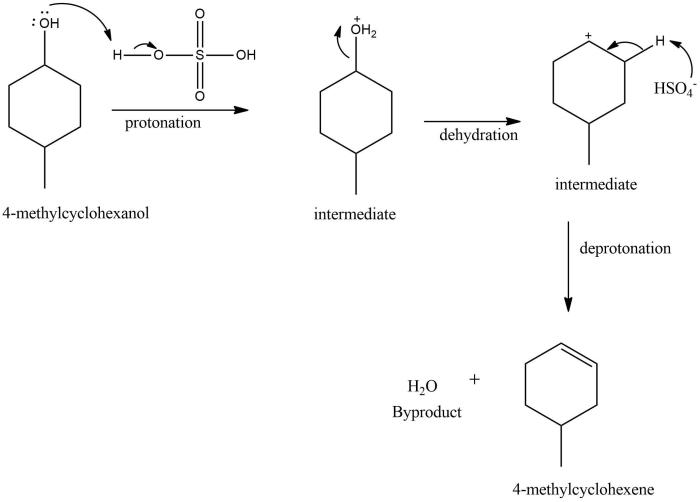
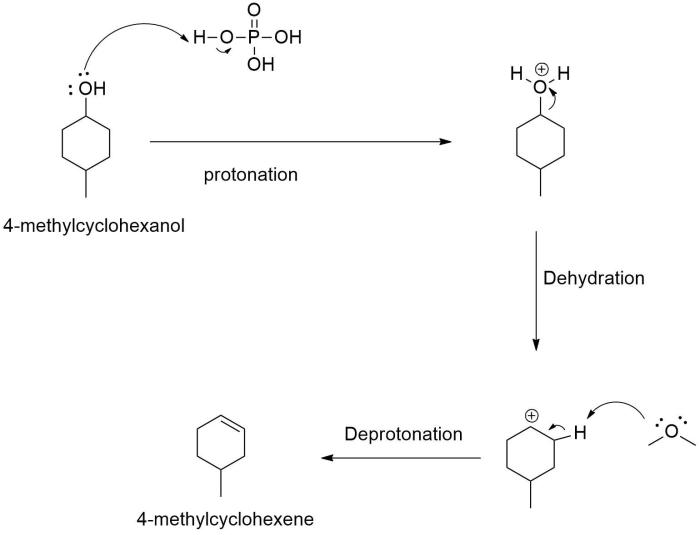
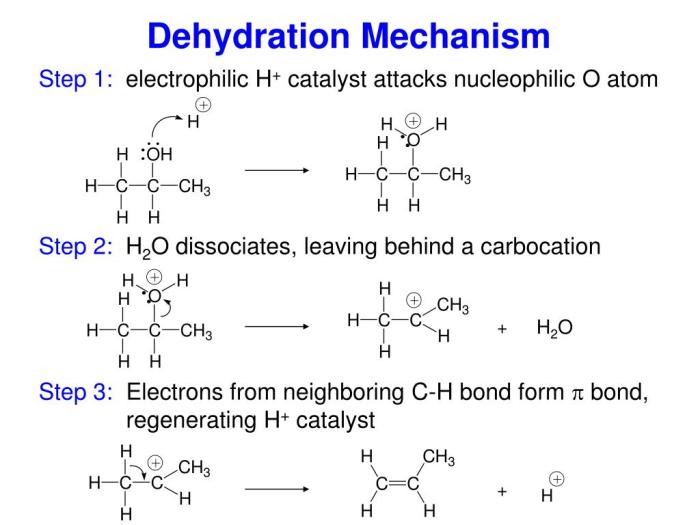
Dehydration is a chemical process that involves the removal of water molecules from a compound. In the case of 4-methylcyclohexanol, dehydration leads to the formation of 4-methylcyclohexene.
The dehydration of 4-methylcyclohexanol is a two-step process. In the first step, the alcohol group is protonated by an acid catalyst, such as sulfuric acid. This protonation makes the alcohol group a better leaving group.
Factors Affecting Dehydration, Dehydration mechanism of 4 methylcyclohexanol
Several factors can affect the dehydration of 4-methylcyclohexanol, including:
- Temperature: The rate of dehydration increases with increasing temperature.
- Catalyst: The type of catalyst used can also affect the rate of dehydration. Acid catalysts, such as sulfuric acid, are typically used for this reaction.
- Solvent: The solvent used can also affect the rate of dehydration. Polar solvents, such as water, can slow down the reaction.
Regioselectivity and Stereoselectivity
The dehydration of 4-methylcyclohexanol is regioselective and stereoselective. The reaction proceeds via a concerted mechanism, which means that the breaking of the O-H bond and the formation of the C=C bond occur simultaneously.
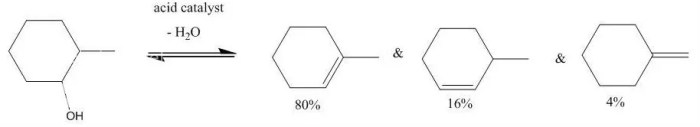
The regioselectivity of the reaction is determined by the stability of the carbocation intermediate. The more stable carbocation is formed, the more likely it is to be the product of the reaction.
The stereoselectivity of the reaction is determined by the orientation of the methyl group. The methyl group can be either cis or trans to the hydrogen atom that is removed. The cis product is more stable than the trans product, so it is the major product of the reaction.
Applications of Dehydration
The dehydration of 4-methylcyclohexanol is a versatile reaction that has a wide range of applications. Some of the most common applications include:
- Production of 4-methylcyclohexene: 4-methylcyclohexene is a valuable intermediate in the production of a variety of chemicals, including polymers, fragrances, and flavors.
- Dehydration of other alcohols: The dehydration of 4-methylcyclohexanol can be used as a model reaction for the dehydration of other alcohols.
- Laboratory techniques: The dehydration of 4-methylcyclohexanol is a useful laboratory technique for the removal of water from organic solvents.
Safety Considerations
The dehydration of 4-methylcyclohexanol is a hazardous reaction that should be carried out with proper safety precautions. Some of the most important safety considerations include:
- Use of a fume hood: The reaction should be carried out in a fume hood to avoid exposure to the toxic fumes that are produced.
- Wear appropriate personal protective equipment: Gloves, safety glasses, and a lab coat should be worn when handling the chemicals used in the reaction.
- Dispose of waste properly: The waste products from the reaction should be disposed of according to local regulations.
FAQ Resource: Dehydration Mechanism Of 4 Methylcyclohexanol
What is the purpose of dehydrating 4-methylcyclohexanol?
Dehydration of 4-methylcyclohexanol aims to remove a water molecule from the molecule, resulting in the formation of a double bond between two carbon atoms. This process is crucial for synthesizing various organic compounds and intermediates used in pharmaceuticals, fragrances, and other industries.
What factors influence the dehydration rate of 4-methylcyclohexanol?
Several factors affect the dehydration rate, including temperature, catalyst type and concentration, solvent polarity, and the presence of impurities. Optimizing these parameters is essential for achieving high conversion and selectivity in the dehydration process.
What are the applications of the dehydration mechanism of 4-methylcyclohexanol?
The dehydration mechanism finds applications in diverse fields. It is employed in the production of terpenes, which are essential components of fragrances and flavors. Additionally, it is utilized in the synthesis of pharmaceuticals, such as ibuprofen and naproxen, and in the preparation of advanced materials like graphene.