Which has the greater mass, a pound of feathers or a pound of gold? It’s a classic riddle that highlights the difference between mass and weight. Mass is a measure of the amount of matter in an object, while weight is a measure of the force of gravity acting on an object.
In this article, we’ll explore the concept of mass, how it’s measured, and some of the applications of mass measurement.
Mass is a fundamental property of matter. It’s a measure of the amount of matter in an object, and it’s independent of the object’s location or the force of gravity acting on it. The SI unit of mass is the kilogram (kg).
One kilogram is equal to the mass of one liter of water at 4 degrees Celsius.
Comparison of Masses: Which Has The Greater Mass
Mass is a fundamental property of matter that measures the amount of substance it contains. It is commonly measured in kilograms (kg), grams (g), or pounds (lb). The mass of an object is a measure of its resistance to acceleration, and it is directly proportional to the amount of matter in the object.
Different objects have different masses. For example, a car has a greater mass than a bicycle, and a planet has a greater mass than a moon. The mass of an object is affected by several factors, including its size, density, and composition.
Factors Affecting Mass
- Size:The larger an object is, the more mass it has. This is because larger objects contain more matter.
- Density:The density of an object is a measure of how much mass it has per unit volume. Objects with a higher density have more mass per unit volume than objects with a lower density.
- Composition:The composition of an object is a measure of the types of atoms and molecules that make it up. Objects with a higher percentage of heavier atoms and molecules have a greater mass than objects with a lower percentage of heavier atoms and molecules.
Measurement Techniques
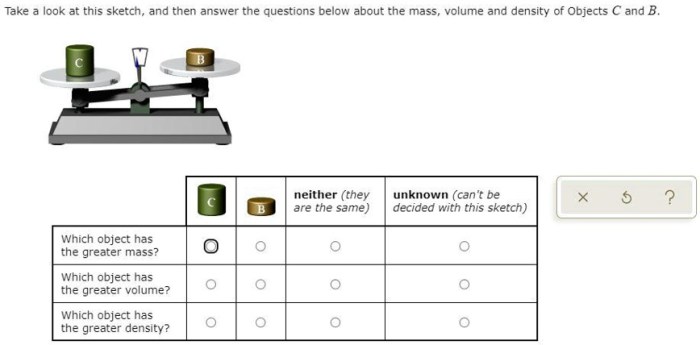
Determining the mass of an object is crucial in various scientific and practical applications. Over the years, scientists and engineers have developed a range of techniques to measure mass with varying degrees of accuracy and precision.
The choice of measurement technique depends on the required accuracy, the size and nature of the object, and the available resources. Some of the most commonly used methods include:
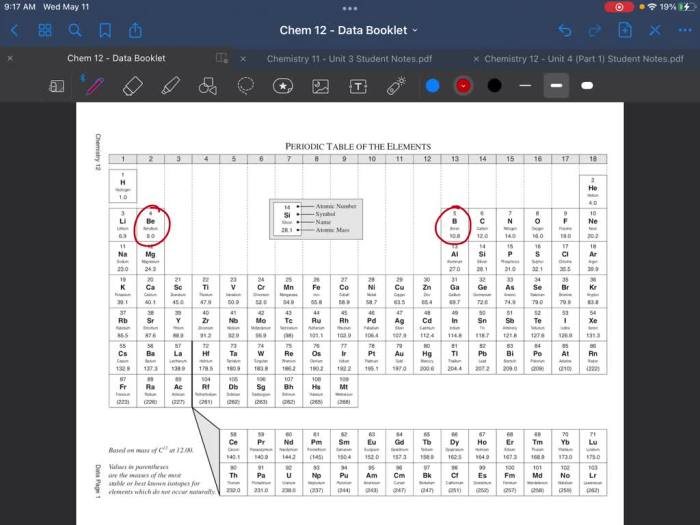
Balance Scales, Which has the greater mass
Balance scales, also known as beam balances, are traditional instruments used to compare the mass of an object to a known mass. They consist of a beam suspended at its midpoint, with pans suspended from each end. The object to be measured is placed on one pan, and known masses are added to the other pan until the beam balances horizontally.
Balance scales are relatively simple to use and provide reasonable accuracy for many applications. However, they can be sensitive to environmental factors such as air currents and vibrations, which can affect the balance point.
Electronic Scales
Electronic scales, also known as digital scales, use electronic sensors to measure the force exerted by an object on a platform. The force is converted into a digital signal, which is then displayed as a mass reading.
Electronic scales are generally more accurate and precise than balance scales, and they are less susceptible to environmental factors. They are also easier to use, as they do not require the addition of known masses.
Gravimetric Analysis
Gravimetric analysis is a technique used to determine the mass of a substance by converting it into a more stable and easily measurable form. This is often used to determine the concentration of a substance in a solution.
The concept of which has the greater mass, like many other psychological concepts, can be further explored in unit 4 ap psychology vocab . There, you’ll find a comprehensive list of terms and definitions that are essential for understanding the complexities of human behavior.
By delving into these materials, you’ll gain a deeper understanding of the factors that influence our thoughts, feelings, and actions, including the concept of which has the greater mass.
In gravimetric analysis, a known volume of the solution is evaporated or precipitated to remove the solvent or other volatile components. The remaining solid is then weighed, and the mass of the substance is calculated based on the known volume of the solution.
Gravimetric analysis is a highly accurate and precise technique, but it can be time-consuming and requires specialized equipment and expertise.
Tips for Obtaining Reliable Mass Measurements
- Use a calibrated scale that is appropriate for the accuracy and precision required.
- Ensure that the scale is placed on a stable and level surface.
- Calibrate the scale regularly according to the manufacturer’s instructions.
- Handle the object to be measured with care to avoid contamination or damage.
- Measure the mass several times and record the average value to reduce errors.
- If possible, use a more accurate and precise measurement technique if the required accuracy is high.
Applications of Mass Measurement
Mass measurement plays a crucial role in numerous fields, from science and engineering to commerce and everyday life. It allows us to quantify the amount of matter in an object, which is essential for understanding its composition, properties, and behavior.
In science, mass measurements are used to determine the composition of materials, calculate their density, and study their physical properties. For example, in chemistry, mass measurements are used to balance chemical equations and determine the molar mass of substances. In physics, mass measurements are used to calculate forces, energy, and momentum.
Engineering
In engineering, mass measurements are essential for designing and constructing structures, machines, and systems. They are used to calculate loads, stresses, and forces, ensuring the safety and efficiency of engineered products. For example, in civil engineering, mass measurements are used to determine the weight of buildings and bridges, while in mechanical engineering, they are used to calculate the mass of moving parts in machines.
Commerce
In commerce, mass measurements are used to determine the weight of goods and materials, which is crucial for pricing, shipping, and inventory management. Accurate mass measurements ensure fair trade practices and prevent disputes between buyers and sellers. For example, in the food industry, mass measurements are used to determine the weight of packaged goods, while in the shipping industry, they are used to calculate shipping costs based on the weight of cargo.
Everyday Life
In everyday life, mass measurements are used in various ways. For example, we use kitchen scales to measure the mass of ingredients when cooking or baking. We use bathroom scales to monitor our weight and track our health progress. And we use postal scales to determine the postage required for mailing letters and packages.
Overall, mass measurement is an essential tool in various fields, providing valuable information about the composition, properties, and behavior of matter. It plays a crucial role in scientific research, engineering design, commercial transactions, and everyday life.
Case Studies and Examples
The determination of mass is a fundamental aspect of various scientific and practical applications. To illustrate the concepts discussed, let’s delve into case studies and examples that showcase the practical significance of mass measurement.
Table of Masses
A comprehensive table comparing the masses of different elements, compounds, and objects provides valuable insights into the relative weights of substances. Such a table can include:
- Masses of elements: Hydrogen (1.008 amu), Helium (4.0026 amu), Oxygen (16.00 amu), etc.
- Masses of compounds: Water (18.0153 g/mol), Carbon dioxide (44.01 g/mol), Sodium chloride (58.44 g/mol), etc.
- Masses of objects: A paperclip (0.5 g), A baseball (145 g), A car (1500 kg), etc.
Flowchart for Determining Mass
A flowchart can effectively demonstrate the step-by-step process of determining the mass of an unknown substance. It typically involves:
- Selecting an appropriate balance or scale
- Calibrating the balance or scale
- Zeroing the balance or scale
- Placing the substance on the balance or scale
- Recording the mass reading
List of Common Objects and Masses
Compiling a list of common objects and their approximate masses can provide a practical reference for everyday use. Such a list might include:
- A sheet of paper (5 g)
- A slice of bread (60 g)
- A cup of coffee (200 g)
- A laptop (2 kg)
- A bag of rice (10 kg)
User Queries
What is mass?
Mass is a measure of the amount of matter in an object.
What is the difference between mass and weight?
Mass is a measure of the amount of matter in an object, while weight is a measure of the force of gravity acting on an object.
How is mass measured?
Mass is measured using a balance scale or an electronic scale.